Marcus Groettrup †02.06.2022 / Guinevere Mathies / Christine Peter
The structural and functional consequences of FAT10 phosphorylation
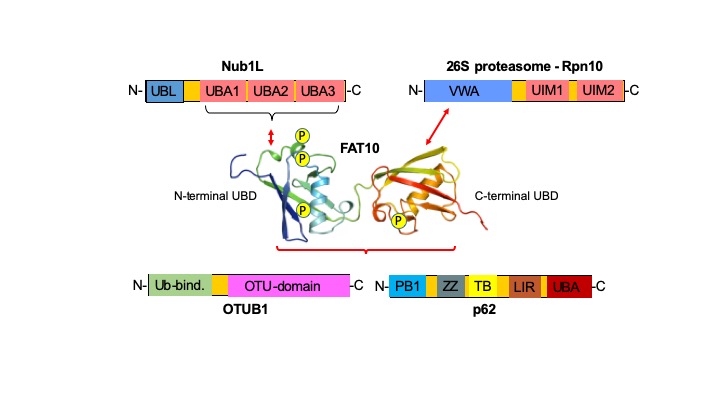
The phosphorylation of ubiquitin has major consequences for its function in mitophagy. Another modifier of the ubiquitin family, designated HLA-F adjacent transcript 10 (FAT10), that also targets conjugated proteins for degradation by the 26S proteasome, becomes phosphorylated in a cytokine-induced manner on three serines and one threonine. In this project three cytokine-activated kinases which phosphorylate FAT10 in vitro and in cells will be validated and further characterized. The consequences of FAT10 phosphorylation for its conjugation, for proteasomal degradation and for binding to p62 and HDAC6 and consecutive localization in aggresomes and autophagosomes will be investigated. The structures and conformational states of wild type, phosphomimetic, and phosphorylated FAT10 as well as two naturally occurring allelic variants will be investigated with magic-angle-spinning NMR and Molecular Dynamics Simulations, respectively. Moreover, patches on the surface of FAT10 required for binding to FAT10 ligands like the proteasome subunit Rpn10 and the linker protein Nub1L will be identified.