Martin Scheffner
Deregulation of protein ubiquitylation by human papillomavirus E6 oncoproteins
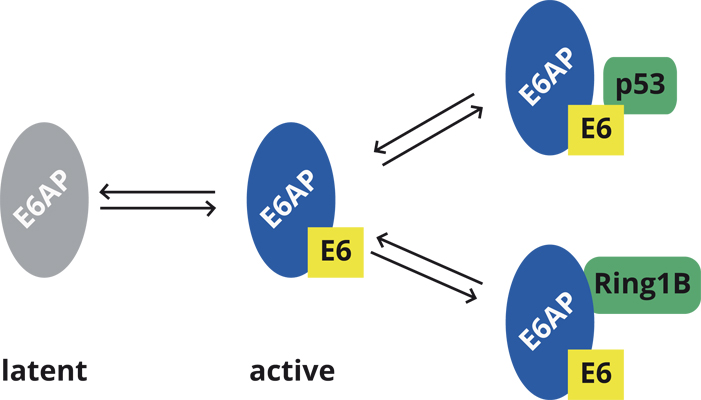
We are studying the interaction of E6 proteins derived from different human papillomaviruses (HPVs) with the cellular E3 ubiquitin ligase E6AP. By a combination of defined ubiquitylation systems and mass spectrometry, we will identify substrates of the E6-E6AP complex and, in consequence, cellular pathways that are affected by E6 and E6AP. Furthermore, we will perform high-throughput-screens to identify small compounds that interfere with the E3 activity of the E6-E6AP complex and that can be used to functionally characterize the E6-E6AP complex in cells. Finally, cell fractionation studies will be used to identify cellular proteins that like the viral E6 proteins stimulate the E3 activity of E6AP. The results obtained will provide insight into the mechanisms by which HPVs reprogram host cells for their own need, and into the function of E6AP in normal, non-infected cells.